
That means there are 11 electrons in a sodium atom. for the element of SODIUM, you already know that the atomic number tells you the number of electrons. Once one shell is full, the next electron that is added has to move to the next shell. Sodium is a classified alkali metal element. As you learn about elements with more than eighteen electrons you will find that shell three can hold more than eight. Sodium is the 11th element in the periodic table and its symbol is ‘Na’.
Na element free#
Sodium is not found free in nature, but sodium compounds are common. Shell number one can only hold 2 electrons, shell two can hold 8, and for the first eighteen elements shell three can hold a maximum of eight electrons. Sodium is the 6th most abundant element in the Earths crust, making up approximately 2.6 of the earth, air, and oceans. They are distinguished by a unique atomic number. The electrons like to be in separate shells/orbitals. An element is a substance that cannot be broken down into a simpler format. In an atom, the electrons spin around the center, also called the nucleus. Stworzenie miej atmosfery i pilnowanie uczniów. Each of those colored balls is an electron. Okrelenie czasu trwania testu (zapisanie na tablicy godziny rozpoczcia i zakoczenia testu). In the next section we're going to cover electron orbitals or electron shells. Sodium (Na) icon structure chemical element round shape. It tells you the mass of one atom, how many pieces are inside, and where it should be placed on the periodic table. Sodium atomic structure has atomic number, atomic mass, electron configuration and energy levels. That box on the left has all of the information you need to know about one element. As a result, when stated in daltons, the numeric value of the atomic mass is roughly equal to the mass number.Check out the blackboard. Protons and neutrons in the nucleus make up nearly all of an atom’s mass, with electrons and nuclear binding energy playing a minor contribution. Although the kilogramme is the SI unit of mass, the atomic mass is sometimes stated in the non-SI unit dalton, which is defined as 1/12 of the mass of a single carbon-12 atom at rest. We get sodium chloride when sodium combines with chlorine (NaCl). The most well-known use of this element is one of the two components of table salt. The mass of an atom is its atomic mass. With the chemical symbol Na and atomic number 11, sodium is a soft, low melting point, silvery-white alkali metal or chemical element in Group 1 or IA of the periodic chart.The number of electrons in an uncharged atom is also equal to the atomic number. It is the same as the charge number of the nucleus. The number of protons in the nucleus of each atom of a chemical element is known as its atomic number.The only way to identify a chemical element is by its atomic number.We know that because sodium’s atomic mass is 23, we can write it as 23 g/mol. The atomic number of sodium is 11, while the number of neutrons in its nucleus is 12.Īs a result, 1 mole of sodium equals 23 grams. Know the atomic mass of sodium, sodium atomic number, chemical properties of sodium and more. In the periodic table, it is the eleventh element. Sodium is a chemical element with symbol Na and atomic number 11. It is the first element of the third periodic period in the periodic table. Each element is identified by the number of protons in its atoms. Sodium is a metal that belongs to the alkali family. There are 118 elements on the periodic table. The following formula was used: Number of neutrons + Number of protons pytanie zadane 22 czerwca 2015 w JavaScript przez Nolandosky Bywalec (2,110 p.) W jaki sposób mona zrobi eby po najechaniu na diva, pojawia si krótki opis.
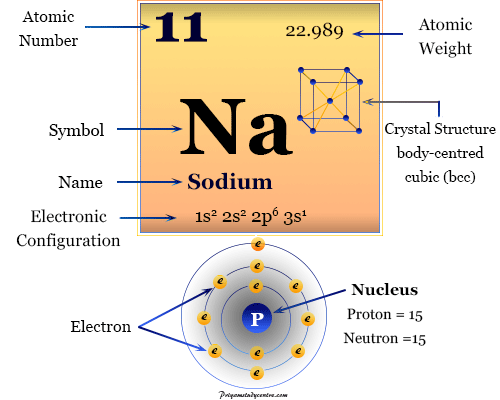

Answer: (b)Įxplanation: The atomic mass of an element can be calculated using the element’s atomic number and the number of neutrons present. Pojawianie si napisu po najechaniu na element. What is the Atomic Mass of Sodium? – Chemistry Q&A (a).


 0 kommentar(er)
0 kommentar(er)
